By Professor Peter G. C. Bedford (2016)
REPRINT FROM: Veterinary Ophthalmology (2017) 20, 2, 98–102 DOI:10.1111/vop.12369
2016 American College of Veterinary Ophthalmologists, Veterinary Ophthalmology
Open-angle glaucoma in the Petit Basset Griffon Vendeen
Peter G. C. Bedford*,†
*Professor Emeritus of Veterinary Ophthalmology, Royal Veterinary College, London, UK; and †Ophthalmology Referrals, 25, Great North Road, Brookmans Park, Herts. AL9 6LB, UK
Abstract
Objectives To report the prevalence and clinical characteristics of an open-angle glaucoma in Petit Basset Griffon Vendeen (PBGV) dogs in the United Kingdom (UK). Animals studied and methods At breed society clinics extending over a 6-year period, 366 dogs of varying ages and both sexes were clinically examined for signs of glaucoma using slit-lamp biomicroscopy, indirect and direct ophthalmoscopy, tonometry, and gonioscopy.
Results The prevalence of glaucoma was 10.4% (38 dogs). Clinical signs of the disease presented from 3 years of age onwards, the commonest initial feature being the elevation of intraocular pressure (IOP) in 15 dogs (39.4%). In addition to elevated IOP, another 13 dogs (34.2%) presented with other features of glaucoma, some with lens subluxation and globe enlargement and all with possible or known vision defects. In the remaining 10 dogs (26.3%), phacodonesis or lens subluxation was observed before subsequent elevation of IOP.
Conclusions High prevalence and similarity to the primary open-angle glaucoma (POAG) seen in the Beagle and Elkhound breeds indicate that an open-angle glaucoma is present in the PBGV in the UK and that this disease may be genetically determined in this breed. Although increased IOP is the commonest early diagnostic feature, lens instability prior to an increase in IOP may be part of the clinical picture.
INTRODUCTION
An inclusive description of glaucoma in the veterinary literature is that of a pathological process usually involving elevation of intraocular pressure (IOP) and resulting in retinal ganglion cell (RGC) and optic nerve degeneration. Several etiologies are involved. In contrast to human glaucoma, where several types of the disease have been described in which the IOP remains at normal or below normal physiological levels, elevation of IOP would appear to be the major risk factor for glaucoma in all other affected animal species. Open-angle glaucoma (POAG) has been extensively described for the Beagle breed(1–3) in which it is inherited as an autosomal recessive trait.(4) Elevation of IOP between 8 and 16 months of age is described as the initial clinical feature, followed by episcleral congestion and mydriasis from approximately 24 months of age, with globe enlargement, lens subluxation, retinal degeneration, and optic disk deformation progressively developing during the following 3 years. The ophthalmoscopic features of the retinal degeneration are increased tapetal reflectivity and blood vessel attenuation, but electrophysiological studies have reported impaired RGC function prior to the appearance of these features.(5–7) The optic disk changes seen are an initial swelling followed much later by cupping with a progressive loss of myelin and closure of blood vessels.8 Significantly, the iridocorneal angle remains open until the end stages of the disease, when the ciliary cleft is collapsed. In some cases, the IOP increases gradually over several years, and although the clinical features are the same, they are slower to present and affected dogs usually remain visual throughout.(9) POAG has been similarly described for the Norwegian Elkhound, the disease being most commonly diagnosed in middle-aged and older dogs.(10–12) It is characterized by an initial small elevation in IOP and an open iridocorneal angle. The lens subluxation which occurs in some affected dogs is considered to be a secondary feature to the IOP rise.
In the Beagle, POAG has been shown to be due to a Gly661Arg missense mutation of the metalloproteinase ADAMTS 10 gene in which a glycine substitution of arginine within its cysteine-rich domain affects microfibril formation and function.(13) The effects are seen in the extracellular matrix (ECM) in both the trabecular mesh- work and the scleral lamina cribrosa, similar to POAG in man where cellular and ECM abnormalities have been described in the same structures.(14) A missense mutation in the exon 9 of the ADAMTS 10 gene is considered to be responsible for POAG in the Norwegian Elkhound, an alanine to threonine change affecting its function in ECM and microfibril composition, suggesting possible defective structure of lens zonule as well as the defects in the aqueous outflow pathway and the scleral lamina cribrosa. (15)
An isolated case of possible open-angle glaucoma in the PBGV was first suspected in 1996 in the UK, and further reports stimulated the breed society to organize an extensive survey upon which this study is based.
ANIMALS STUDIED AND METHODS
All dogs were examined under the British Veterinary Association/Kennel Club/International Sheepdog Association Eye Examination Scheme and the health directives of the Basset Griffon Vendeen Club. Owners’ informed consent was acquired for all dogs in the study and the subsequent publication of results.
Three hundred and sixty-six adult PBGV dogs aged between 8 months and 6 years were examined by the author over a 6-year period from 2009 to 2014. There were 242 females (196 entire) and 124 males (93 entire). Most dogs were seen only once, but 108 dogs including 11 receiving glaucoma treatment were re-examined on at least two occasions. All the dogs were examined in dark room facilities using slit-lamp biomicroscopy (Keeler, PSL Classic, Windsor, UK), both indirect and direct ophthalmoscopy (Keeler), applanation tonometry (Tono-Pen Vet, Medtronic, Jacksonville, FL, USA), and gonioscopy (Barkan lens, Medical Workshop, Groningen, Netherlands). Tonometry and gonioscopy using 0.5% proxymetacaine HCl (Minims, Laboratoire Chauvin, Aubenas, France) were completed before inducing mydriasis with 1% tropicamide (Mydracyl 1%, SA Alcon-Couvreur, Puurs, Belgium). Slit-lamp biomicroscopy was completed before and after mydriasis and ophthalmoscopy completed after mydriasis. Vision assessment was based on history, menace and dazzle reflexes, and, whenever possible and where indicated, maze performance.
All 28 dogs presenting with increased IOP, with or without other signs of glaucoma, and the 10 dogs presenting with phacodonesis or lens subluxation in normotensive eyes were DNA sampled using standard buccal mucosal swabs (Animal Health Trust, Newmarket, United Kingdom) taken at the time of the initial examination. The commercially available tests for the ADAMTS 10 mutation responsible for POAG in the Beagle13 and the ADAMTS 17 mutation responsible for primary lens luxation in several breeds(16,17) were completed by the Kennel Club Genetics Centre at the Animal Health Trust (http:// www.aht.org.uk/genetics_test.html).
RESULTS
Clinical findings
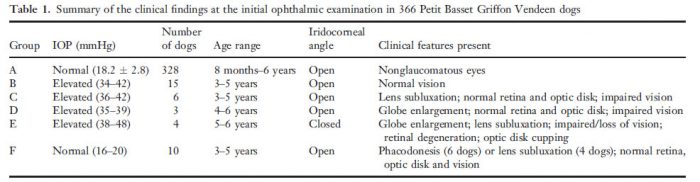
In addition to the normal, five clinical presentations associated with glaucoma could be described at the primary examination, the details of which are found in Table 1 (summary of the clinical findings at the initial ophthalmic examination in 366 Petit Basset Griffon Vendeen dogs). There were 328 normotensive dogs in which the iridocorneal angles were open and the pectinate ligaments considered to be normal (group A, Table 1). All quadrants of the angle could be sufficiently examined using the combination of goniolens and biomicroscope. There was considerable variation in the amount of pigmentation in both pigment bands and although individual pectinate fibers varied in thickness, fibrae latae and laminae (sheeting) were not seen. (Fig. 1). The average normal IOP for this group was 18.2 mmHg, with a standard deviation of ±2.8 mmHg.
Twenty-eight dogs presented with elevated IOP values of between 34 and 48 mmHg (groups B to E, Table 1). The iridocorneal angles were open without pectinate ligament abnormality in 24 of these dogs (groups B to D) (Fig. 2). Other features of glaucoma were seen in groups C and D (see Table 1), and in the 4 dogs in group E, angle closure was present accompanied by globe enlargement, lens subluxation, retinal degeneration, and optic disk cupping (Fig. 3).
Table 1. Summary of the clinical findings at the initial ophthalmic examination in 366 Petit Basset Griffon Vendeen dogs
In a further 10 dogs (group F, Table 1), the initial presenting clinical features were either phacodonesis or lens subluxation seen unilaterally (three dogs) or bilaterally (six dogs) in normotensive eyes (Fig. 4). The phacodonesis was usually accompanied by iridodonesis, and where subluxation was present, the early aphakic crescent was always seen dorsally or dorsolaterally. Within the crescent, either intact elongated zonular fibers were seen or the lens equator was irregularly darkened or highly reflective. In two dogs, possible vitreal or zonular material was seen within the pupillary aperture in the region of the aphakic crescent. The iridocorneal angles were open and without pectinate ligament abnormality. Vision was considered to be normal in these 10 dogs at the initial examination:
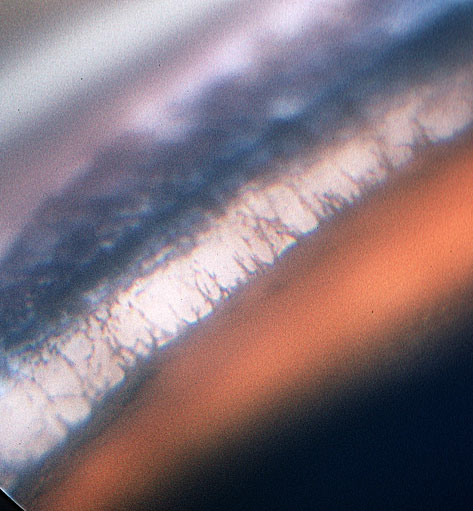
Figure 1. An open iridocorneal angle in a 4-year-old normotensive PBGV, dorsolateral quadrant, right eye, showing normal pectinate fibers and pigmentation of the pigment bands. The pupil is semi-dilated.
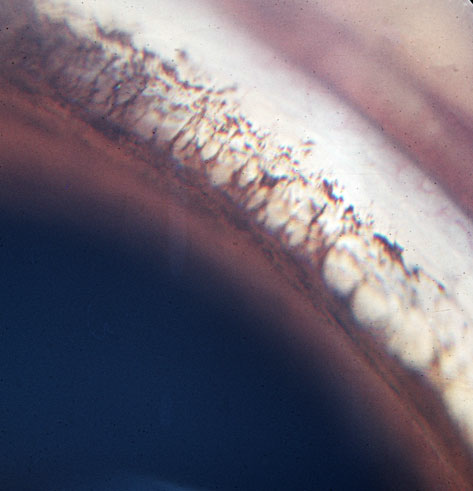
Figure 2. An open iridocorneal angle in a 5-year-old PBGV with glaucoma, dorsolateral quadrant, left eye. There is no pigmentation of the pigment bands, and the pupil is dilated.

Figure 3. Closure of the iridocorneal angle in a 6-year-old PBGV with glaucoma and globe enlargement. Dorsolateral quadrant, left eye.
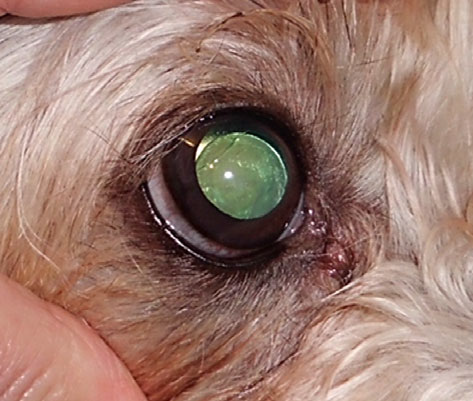
Figure 4. The normotensive right eye of a 4-year-old PBGV exhibiting a dorsolateral aphakic crescent.
Subsequently, elevated IOP values were recorded in all 10 dogs and despite subsequent treatment to restore and maintain normotension, six developed defective vision.
Lens subluxation was most marked in the four dogs in group E (Table 1), but as the lenses seemingly remained attached to the anterior vitreous in all the dogs throughout the course of the study, complete luxation with the lens either displacing posteriorly into the vitreous or anteriorly into the pupil or the anterior chamber was not seen. Patchy peripapillary or generalized retinal degeneration was seen in the 4 dogs presenting with globe enlargement at the initial presentation, two of which were clinically blind (group E, Table 1). Retinal changes were subsequently seen in nine medically treated dogs (Xalatan, Pfizer, Havant, UK) from groups B and F for which regular re-examination was possible. In another two dogs from group B in which shunt surgery maintained normotension, retinal degeneration progressively developed during an 18-month follow-up period. In these 11 dogs, tapetal hyper-reflectivity was seen initially in the peripapillary and mid-tapetal zones of the fundus, but gradually the hyper-reflectivity became more generalized and was accompanied by superficial retinal blood vessel attenuation. An initial thinning of the peripheral disk tissue was seen on ophthalmoscopy using a red-free filter in seven of these 11 dogs: It was accompanied by papillary blood vessel attenuation, but the subsequent cupping recorded in only three dogs re-examined at the end of the study was minimal and the iridocorneal angles were open at this stage.
When corneal edema was present (groups D and E, Table 1), it was mild and the degree of episcleral congestion was variable (groups C, D and E, Table 1). Marked optic disk changes were only seen in the four dogs in group E (Table 1), the disks appearing smaller than normal with cupping rendering them dark in appearance.
Twenty-five females (10.3%) and 13 males (10.4%) were affected, with no significant relationship found between gender and the disease (chisquare (2, N = 366) = 0.002, P > 0.05).
All 38 affected dogs tested negatively for both the ADAMTS 10 and the ADAMTS 17 mutations.
Among the 366 dogs, there were several incidental findings including 58 dogs with minor persistent pupillary membrane remnants, 19 dogs with distichiasis, six dogs with corneal scarring, and two dogs with corneal lipidosis.
DISCUSSION
The glaucoma seen in the PBGV in this study is substantially similar to that previously described for POAG in both the Beagle and the Norwegian Elkhound. Iridocorneal angles were open in all but the four dogs with marked globe enlargement, and the commonest initial clinical sign of disease was the increase in IOP seen in 39.4% of affected dogs. However, phacodonesis or lens subluxation prior to a subsequent elevation of IOP was noted in 26.3% of initially normotensive dogs. These results beg the obvious question: in this breed are there two diseases with two distinct etiologies for which the common end point is glaucoma or just one disease in which both lens zonule and the aqueous drainage pathway are affected? Comparison with POAG in the Beagle is valid in an examination of the role of increased IOP as the initiating factor in the subsequent development of the pathological features of glaucoma. Undoubtedly sustained IOP elevation will cause both structural damage and functional damage, but in Beagle POAG disturbance of the IOP seemingly may be just one part of a complex of changes contributing to the overall picture, rather than the initiating cause of the glaucoma. PERG studies have shown that there can be a decrease in RGC function before a rise in IOP.(5–7) Reduction in both orthograde and retrograde axoplasmic flows, reduced blood vessel velocity at the scleral lamina cribrosa, and impairment of orbital microcirculation all similarly occur before pressure rises.(8,18–20)
The traditional view of lens subluxation in POAG in the Beagle is that it is a function of globe enlargement when the zonular fibers are stretched to the point of partial disruption, but Komaromy et al.(21) have recently reported lens luxation prior to increased IOP. Stretching and tearing of the zonular fibers were recorded as early as 6 months of age in normotensive eyes. This is an important observation for the affected dogs were homozygous for the ADAMTS 10 mutation responsible for POAG in the Beagle, suggesting that this mutation may have an effect on extracellular matrix in several parts of the eye. Elevation of IOP was the earliest indication of glaucoma in most of the affected PBGV dogs, but the presence of phacodonesis or lens subluxation prior to the subsequent elevation of IOP in normotensive eyes may reflect lens zonular abnormalities similar to those seen in the Beagle with POAG. The disease in the PBGV bears many similarities to the human Weill–Marchesani syndrome in which lens luxation is a predominant feature, together with short stature. Mutations of both the ADAMTS 10 and ADAMTS 17 genes are involved.(22) Canine primary lens luxation (PLL) has been shown to result from a different mutation in the ADAMTS metalloproteinase family, a single nucleotide substitution of intron 10 of ADAMTS 17. (16) However, the PLL classically seen in the terrier breeds is more typically associated with acute onset, secondary angle closure due to pupillary block, in sharp contrast to the chronic, gradually progressive course of the disease in the PBGV. Although none of the glaucomatous PBGV dogs tested positive for either the previously identified Beagle ADAMTS 10 or terrier ADAMTS 17 mutations, the possibility of other mutations in either of these genes, or related genes, cannot be excluded.
There were several unavoidable limitations to this study, the greatest of which were the practicalities of subsequent regular re-examination for all the dogs involved. Assessment of IOP and its normal value are essential factors in the diagnosis of open-angle glaucoma simply because the early elevations are small and initially asymptomatic. The same examiner and tonometer were employed to reduce operator error, but despite most of the examinations being conducted during the middle 5 or 6 h of the day, the readings could not take into account diurnal variation or other factors that can influence results. IOP estimations are not constant even in the same dog, and ideally, several daily recordings are preferable to single examinations. Diurnal variation may be as much as 3 or 4 mmHg. in normotensive dogs, but differences of up to 10 mmHg. have been recorded in POAG patients.(23) Tonography, ultrasonography, color Doppler technology, water provocation, and corticosteroid challenge tests were not practical in the context of this study. Tonography could have identified a reduced aqueous outflow facility in those dogs predisposed to open-angle glaucoma,(10,11) and ultrasonography may have demonstrated possible changes within the ciliary cleft which could impair aqueous outflow.(24) Electroretinography may identify retinal functional abnormalities in advance of ophthalmoscopic abnormalities in glaucoma. Subtle ophthalmoscopic signs of retinal and optic disk degeneration were observed, but the nature of this clinical study did not permit histological confirmation of the extent or nature of the underlying pathology.
The size of the PBGV population in the UK is unknown, but official Kennel Club registrations for the last 10 years total 1565 dogs. This would suggest that the 366 dogs examined in this study were representative of the breed at large and that the prevalence figure and the clinical features of a possible POAG in this breed are reliable. Inclusive of those dogs that presented with phacodonesis or lens subluxation prior to ocular hypertension, the survey records an overall glaucoma period prevalence of 10.4% for the breed.. The slow course of early disease without the dramatic features of an angle-closure glaucoma means that unless there is regular ocular examination of young to middle-aged dogs, owners may only become aware of the disease when sight impairment or globe enlargement becomes noticeable. This high prevalence of a disease clinically similar to the POAG inherited in the Beagle and Elkhound breeds strongly supports speculation that it is also genetically determined. High prevalence and late clinical diagnosis combine to complicate current breeding programs for the PBGV and will render disease control extremely difficult until a possible mode of inheritance is determined and a DNA test developed.
REFERENCES
- Gelatt KN, Peiffer RL, Gwin RM et al. Clinical manifestations of inherited glaucoma in the Beagle. Investigative Ophthalmology & Visual Science 1977; 16: 1135–1148.
- Peiffer RL, Gelatt KN. Aqueous humour outflow in Beagles with inherited glaucoma: gross and light microscopic observations of the iridocorneal angle. American Journal of Veterinary Research 1980; 41: 861–867.
- Gelatt KN, Gum GG, Gwin RM et al. Primary open angle glaucoma: inherited open angle glaucoma in the Beagle. American Journal of Pathology 1981; 102: 292–295.
- Gelatt KN, Gum GG. Inheritance of primary open angle glaucoma in the Beagle. American Journal of Veterinary Research 1981; 42: 1691–1693.
- Ofri R, Dawson WW, Gelatt KN. Visual resolution in normal and glaucomatous dogs determined by pattern electroretinogram. Veterinary & Comparative Ophthalmology 1993; 3: 111–116.
- Ofri R, Dawson WW, Foli K et al. Primary open angle glaucoma alters retinal recovery from a thiobarbiturate: spatial frequency dependence. Experimental Eye Research 1993; 56: 481–488.
- Ofri R, Samuelson DR, Strubbe DT et al. Altered retinal recovery and optic nerve fibre loss in primary open angle glaucoma in the Beagle. Experimental Eye Research 1994; 58: 245–258.
- Brooks DE. Confocal scanning laser ophthalmoscopy. Transactions of the American College of Veterinary Ophthalmology 1996; 27: 130.
- Gelatt KN, Brooks DE. The canine glaucomas. In: Veterinary Ophthalmology. (ed. Gelatt KN) Lippincott Williams and Wilkins, Baltimore, 1999; 701–754.
- Bjerkas E, Peiffer RL, Ekesten B. Primary glaucoma in the Norwegian Elkhound. Transactions of the American College of Veterinary Ophthalmology 1994; 25: 74.
- Ekesten B, Bjerkas E, Kongsengen K et al. Primary glaucoma in the Norwegian Elkhound. Veterinary & Comparative Ophthalmology 1997; 7: 14–18.
- Oshima Y, Bjerkas E, Peiffer RL. Ocular histopathologic observations in Norwegian Elhounds with primary open-angle, closed cleft glaucoma. Veterinary Ophthalmology 2004; 7: 185–188.
- Kuchtey J, Olson LM, Rinkoski T et al. Mapping of the disease locus and identification of ADAMTS 10 as a candidate gene in a canine model of primary open angle glaucoma. PLoS Genetics 2010; 7: e1001306.
- Gottanka J, Johnson DH, Martus P. Severity of optic nerve damage in eyes with POAG is correlated with changes in the trabecular meshwork. Journal of Glaucoma 1997; 6: 123–132.
- Ahonen SJ, Kaukonen M, Nussdorfer FD et al. A novel missense mutation in ADAMTS 10 in Norwegian Elkhound glaucoma. PLoS ONE 2014; 9(11): e111941.
- Farias FH, Johnson GS, Taylor JF et al. An ADAMTS 17 splice donor site mutation in dogs with primary lens luxation. Investigative Ophthalmology and Visual Science 2010; 51: 4716– 4721.
- Gould D, Pettit L, McLaughlin B et al. ADAMTS 17 mutation associated with primary lens luxation is widespread among breeds. Veterinary Ophthalmology 2011; 14: 378–384.
- Samuelson DA, Williams LW, Gelatt KN et al. Orthograde rapid axoplasmic transport and ultrastructural changes of the optic nerve. Part 2. Beagles with primary open angle glaucoma. Glaucoma 1993; 5: 174–184.
- Gelatt-Nicholson KJ, Gelatt KN, Mackay EO et al. Comparative Doppler imaging of the ophthalmic vasculature in normal Beagles and Beagles with inherited open angle glaucoma. Veterinary Ophthalmology 1999; 2: 97–105.
- Bito LZ. The physiology and pathophysiology of intraocular fluids. In: The Ocular and Cerebrospinal Fluids. (eds Bito LZ, Davson H, Fenstermacher JD) Academic Press: New York, 1977; 273–289.
- Komaromy EM, Storey ES, Iwabe S et al. New insight on an old disease: primary abnormalities in the extracellular matrix of lens zonules and sclera in Beagles with inherited open-angle glaucoma. Proceedings of the ECVO meeting, Trieste 2012; P. 43.
- Morales J, Al-Sharif L, Khalil DS et al. Homozygous mutations of ADAMTS 10 and ADAMTS 17 cause lenticular myopia, ectopia lentis, glaucoma, spherophakia and short stature. American Journal of Human Genetics 2009; 85: 558–568.
- Gelatt KN, Gum GG, Barrie KP et al. Diurnal variations in intraocular pressure in normotensive and glaucomatous Beagles. Glaucoma 1981; 3: 121–124.
- Samuelson DA, Gum GG, Gelatt KN. Ultrastructural changes in the aqueous outflow apparatus of Beagles with inherited glaucoma. Investigative Ophthalmology and Visual Science 1989; 30: 550–561.
© 2016 American College of Veterinary Ophthalmologists, Veterinary Ophthalmology, 20, 98–102
Leave A Comment